Transition Metal-Catalyzed Formation of Boron−Nitrogen Bonds: Catalytic Dehydrocoupling of Amine-Borane Adducts to Form Aminoboranes and Borazines | Journal of the American Chemical Society

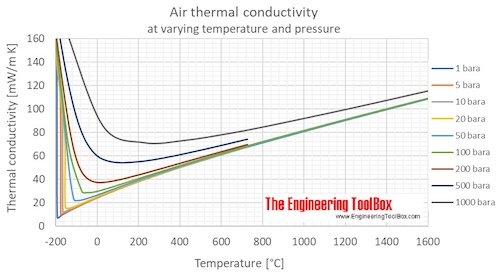
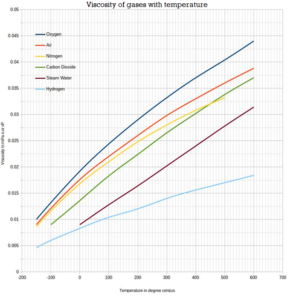
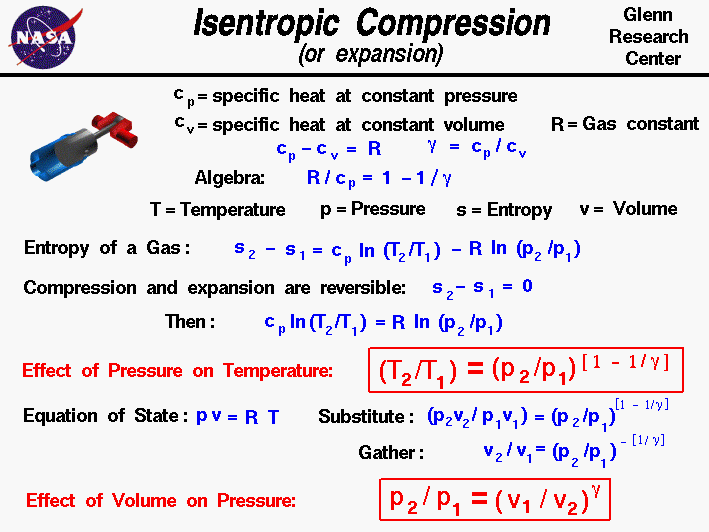
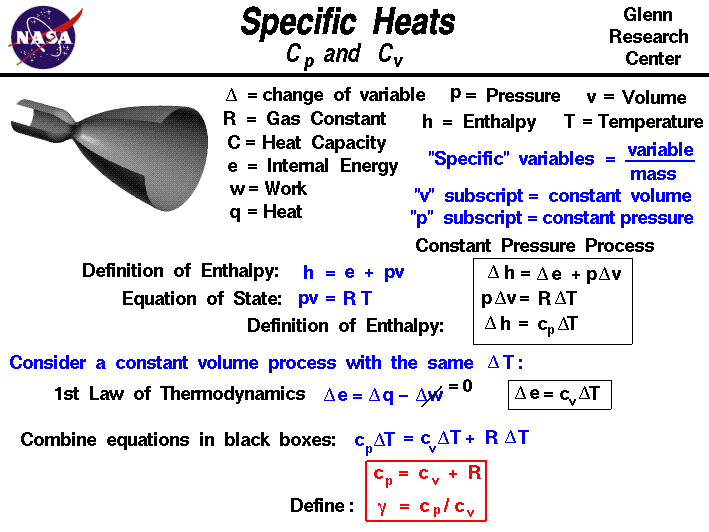
If Cp and Cy denote the specific heats of nitrogen per unit mass constant pressure and constant volume respectively, then Cp - Cv = 28R Cp - CV =R/28 Cp - Cy=R/14 •
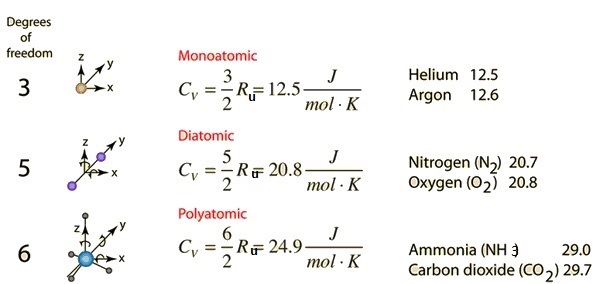
What are the CP and CV of a gas if that gas has n degrees of a freedom ratio of specific heat? - Quora

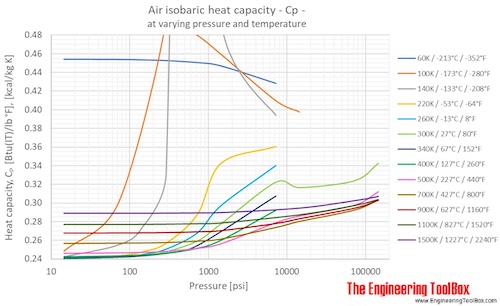
A global equation-of-state model from mathematical interpolation between low- and high-density limits | Scientific Reports

Colorimetric Fluoride Ion Sensing by Polyborylated Ferrocenes: Structural Influences on Thermodynamics and Kinetics | Inorganic Chemistry
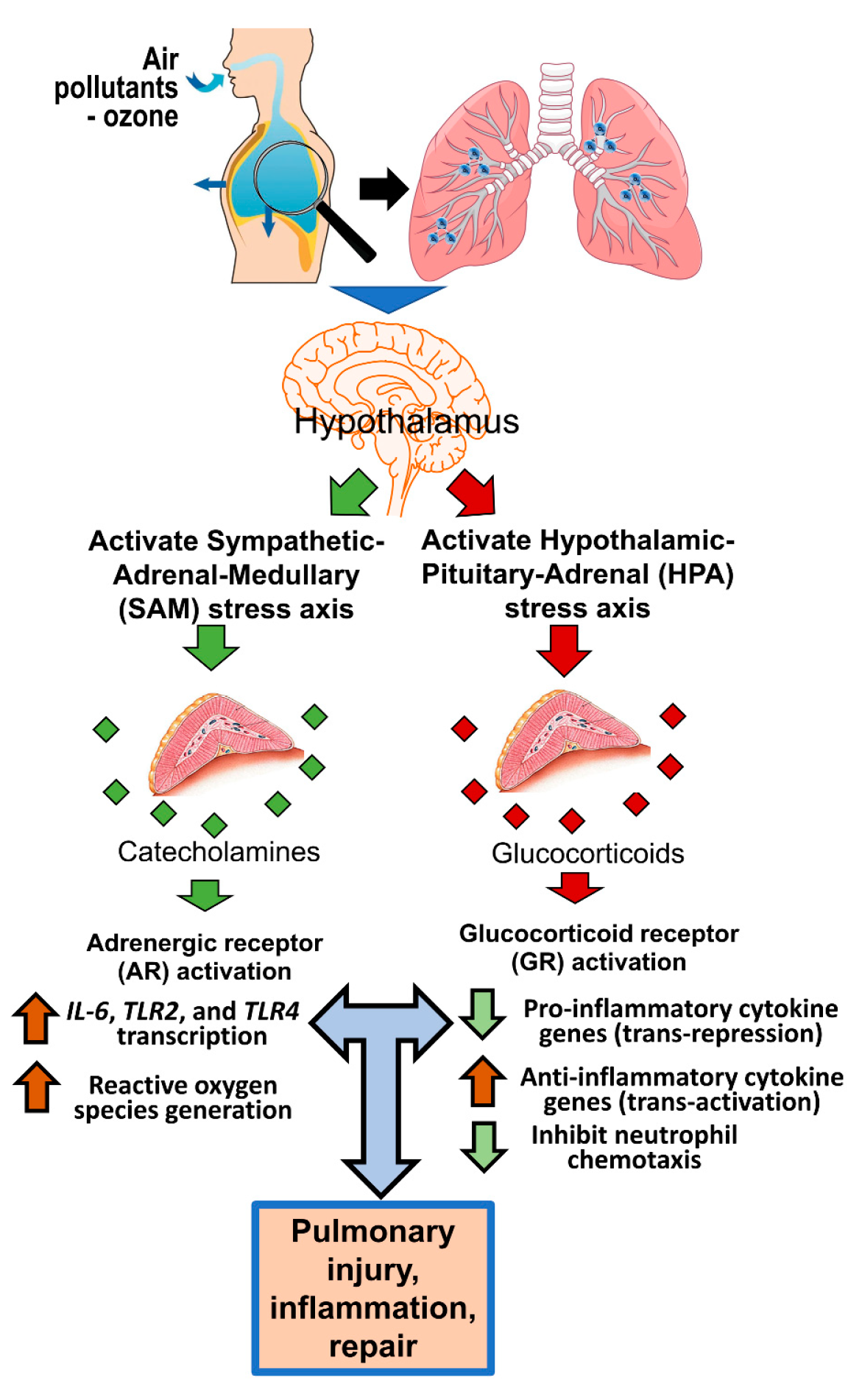
Toxics | Free Full-Text | Adrenergic and Glucocorticoid Receptors in the Pulmonary Health Effects of Air Pollution

IJMS | Free Full-Text | Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation







![Slammedenuff Air Suspension [MAKE] Slammedenuff Air Suspension [MAKE]](http://slammedenuffshop.com/cdn/shop/products/slammedenuff-air-suspension-slammedenuff-air-suspension-make-33289619308714_1200x1200.png?v=1652949381)